Artificial Resuscitators are mechanically or manually operated devices that serve as breathing supplements or substitutes to force air and/or oxygen mixtures into a patient’s lungs. These devices range from simple bag-valve masks (BVMs) used in prehospital settings to highly advanced ventilation devices incorporated as part of intensive care units.
In essence, the artificial resuscitator will encompass the functions of accurately modulating the volume, pressure, and concentration of oxygen for ventilation. In the emergency health sector, rapid and adequate ventilation support can significantly contribute to a patient’s survival during respiratory failure or arrest.
Key technical considerations include:
- Airflow Control Mechanisms: Manual resuscitators rely on operator compression, whereas advanced models employ electronic controls for synchronized ventilation.
- Pressure Regulation: To prevent lung injury, devices incorporate pressure limiters or feedback systems.
- Oxygen Integration: Many artificial resuscitators can be coupled with external oxygen sources to enhance oxygen delivery.
The Significance of Artificial Resuscitators in Modern Healthcare
Artificial Resuscitators have a critical role in the management of respiratory distress due to varied etiology such as trauma, heart failure, neurological disorders, and ARDS (Acute Respiratory Distress Syndrome). They also provide the foundation of care in the surgical and intensive care units.
The relevance of these devices is emphasized in cases of public health crises, including those involving respiratory viruses during a pandemic outbreak. The focus on the availability and usage of artificial resuscitators has become an issue of great concern among health facilities globally.
Key implications of artificial resuscitator use include:
- Reduction of Mortality in Respiratory Failure: Timely ventilation support mitigates hypoxia and organ dysfunction.
- Facilitation of Surgical Procedures: Controlled ventilation enables safe administration of anesthesia.
- Support in Critical Care: Continuous respiratory support allows stabilization and recovery in critically ill patients.
GPC Medical Ltd.: Prominence in Artificial Resuscitator Manufacturing
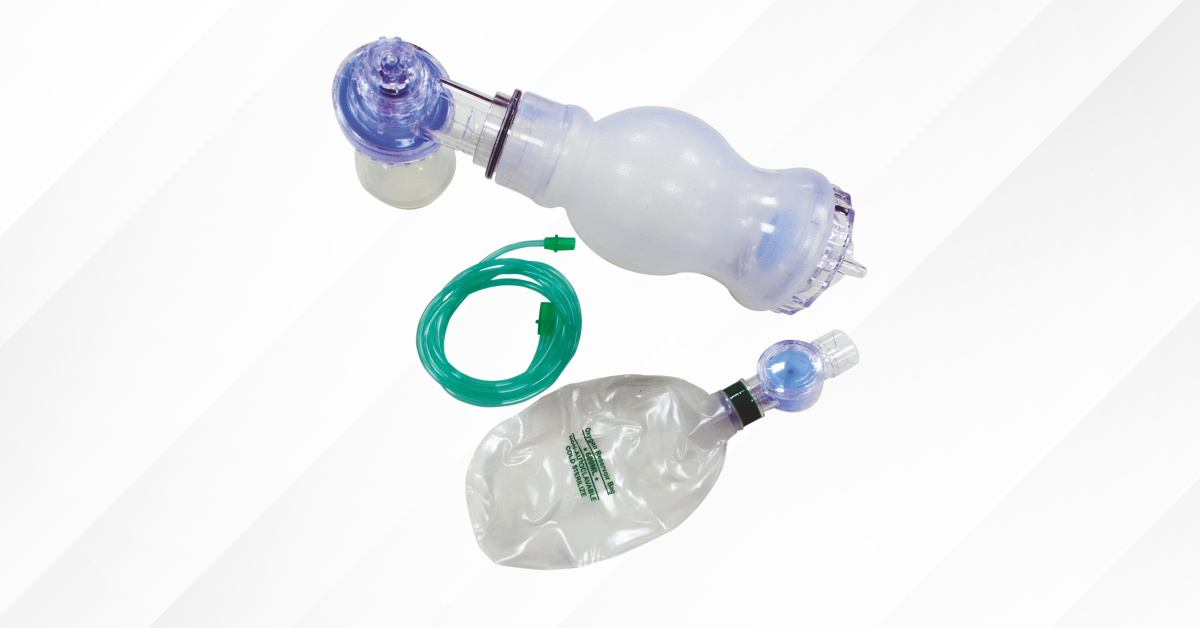
GPC Medical Ltd. has established itself as a prominent Artificial resuscitator manufacturer and Artificial resuscitator exporter with a global footprint. The company has strategically invested in state-of-the-art manufacturing facilities, research and development (R&D), and rigorous compliance frameworks to deliver high-performance resuscitation equipment. The artificial resuscitator is engineered for reliable emergency ventilation, featuring a 250 ml to 1600 ml capacity for adult, infant and child emergencies. These are supplied with a 2-meter oxygen tubing for flexible clinical use. The device incorporates silicone rubber inner and outer bags, along with a silicone override cap, valve disc, strap, and AO mask for durability, comfort, and effective sealing. The patient valve housing is manufactured from polysulphone (PSU), while the patient connector combines PSU and silicone rubber for strength and flexibility. Safety and performance are further supported by a pressure-limiting valve made of PSU and stainless steel. The inlet valve housing, two-part screw cap, and connector are constructed from polyacetal (POM), ensuring precision and wear resistance, while the expiration connector is made of polypropylene (PP) for lightweight efficiency.
Capabilities and Strategic Initiatives
GPC Medical Ltd.’s manufacturing strategy emphasizes:
- Advanced Production Systems: Utilization of automated assembly lines and precision engineering ensures consistency and scalability.
- Custom Development: Modular design methodologies facilitate customization for diverse clinical needs and regulatory environments.
- Global Distribution Networks: A robust logistics infrastructure supports timely export and market access across continents.
Quality Assurance in Artificial Resuscitator Production
Quality assurance involves a set of well-structured processes that enable consistency and reliability by meeting regulatory, safety, and performance standards. Given the life-critical application of these devices, QA frameworks have to be robust, evidence-based, and continually validated. GPC Medical Ltd.’s commitment to quality control (QC) reflects an organizational ethos that prioritizes patient safety and regulatory compliance.
- Standards Compliance: Adherence to international regulatory standards such as ISO 13485 (Medical Devices — Quality Management Systems).
- Design Verification and Validation: Ensuring that products meet intended use specifications through rigorous laboratory and clinical simulations.
- Traceability and Documentation: Comprehensive record-keeping from raw materials to final product release facilitates accountability and corrective action if needed.
- Final Product Testing: Functional testing of each artificial resuscitator unit to verify performance criteria such as pressure regulation, flow integrity, and mechanical resilience.
Economic Considerations: Price and Market Dynamics
The cost of raw materials, technological sophistication, costs associated with regulatory compliance, and production scale are among the factors that influence the price of artificial resuscitators. For healthcare purchasers ranging from emergency response units to large hospital networks, the cost of an artificial resuscitator must strike a balance between affordability and uncompromised quality.
GPC Medical Ltd. employs cost-effective production techniques that optimize material utilization, streamline assembly processes, and leverage economies of scale. These efficiencies support competitive pricing while upholding strict performance requirements.
Future Directions and Conclusion
The need for artificial resuscitators will inevitably rise as healthcare continues to deal with difficult respiratory issues, such as pandemics and chronic pulmonary diseases. Continuous advancements in automation, materials science, and sensor technology have the potential to improve clinical versatility, user safety, and device efficacy.
GPC Medical Ltd. is a prime example of an innovative artificial resuscitator manufacturer and exporter that combines strict quality control, adaptable product development, and international market participation. The company supports the global demand for increasing access to dependable respiratory support technologies through investments in manufacturing excellence and strategic partnerships.